Manage research ethics with confidence
Streamline ethics applications, approvals, and ongoing oversight with clear audit trails and compliance support.

The Challenge
Institutions struggle with scattered researcher profiles, duplicated publication records, and increasing pressure to demonstrate impact and meet national reporting requirements. Manual processes consume time, increase errors, and create stress during reporting cycles.
The Solution
Vidatum Research brings researcher profiles, outputs, projects, and impact data into one connected system. Automated data enrichment and integrations reduce duplication, improve accuracy, and provide a reliable foundation for reporting, benchmarking, and strategic planning.
The Challenge
The Solution
Key benefits
A clear set of outcomes that support reliable research reporting, stronger data quality, and consistent institutional insight.
Structured Ethics Applications
Ensure consistent, complete ethics submissions using configurable forms that capture required information and supporting documentation from the start.
Clear Review Workflows
Replace email-based reviews with transparent workflows that support committee reviews, conditions, and decisions with clear status visibility.
Amendment & Renewal Tracking
Manage amendments, renewals, and ongoing monitoring in one place, supporting continuous ethics oversight throughout the research lifecycle.
Built-In Audit Trails
Maintain a complete record of submissions, reviews, decisions, and changes to support audits, inspections, and internal governance reviews.
Centralised Ethics Records
Create a single source of truth for ethics data, improving coordination between researchers, committees, and research administration teams.
Change History Visibility
Use AI-assisted comparison to highlight updates between submission rounds, helping reviewers focus on changes, respond to conditions, and assess revisions more efficiently.
Detail & Depth
Support separate workflows and reporting for HRECs, animal ethics, biosafety, and other committees within one institutional system.
Dynamically show or hide questions based on previous answers, risk category, and research methodology, ensuring relevance and completeness.
Manage conflicts of interest and facilitate peer review with secure, anonymised distribution of protocols to internal or external reviewers.
Track every amendment, resubmission, and renewal in a full version history, linked directly to the original application.
A quick look at Vidatum Ethics
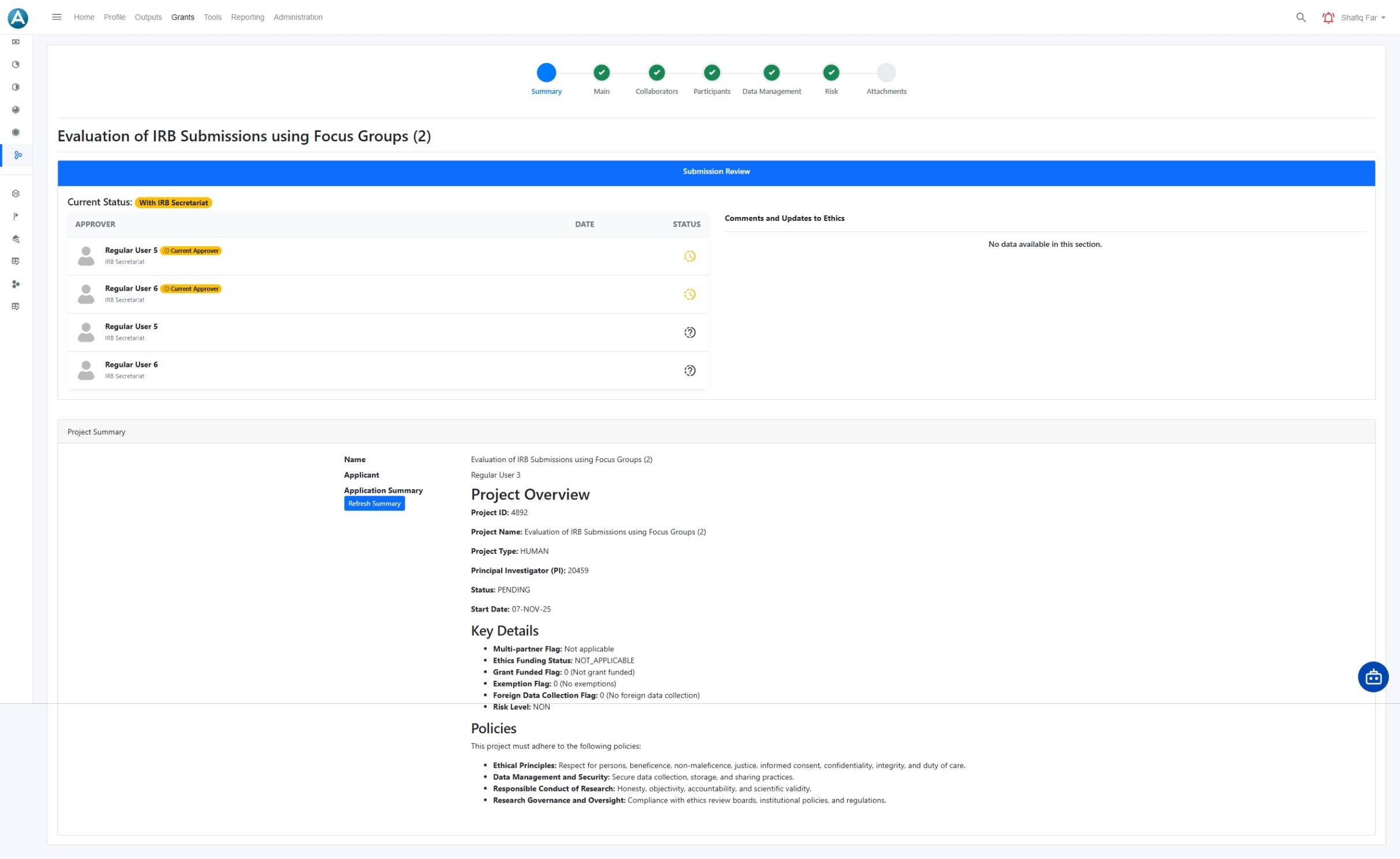
Ethics summary and governance overview
A consolidated ethics summary gives administrators and committees a clear view of project context, risk indicators, and governance requirements. This supports informed decisions and audit readiness without manual collation.
- Single page overview of ethics project details
- Visibility of risk level and compliance flags
- Linked policies and governance requirements
- Clear record of approvals and conditions
- Audit ready view for internal and external review
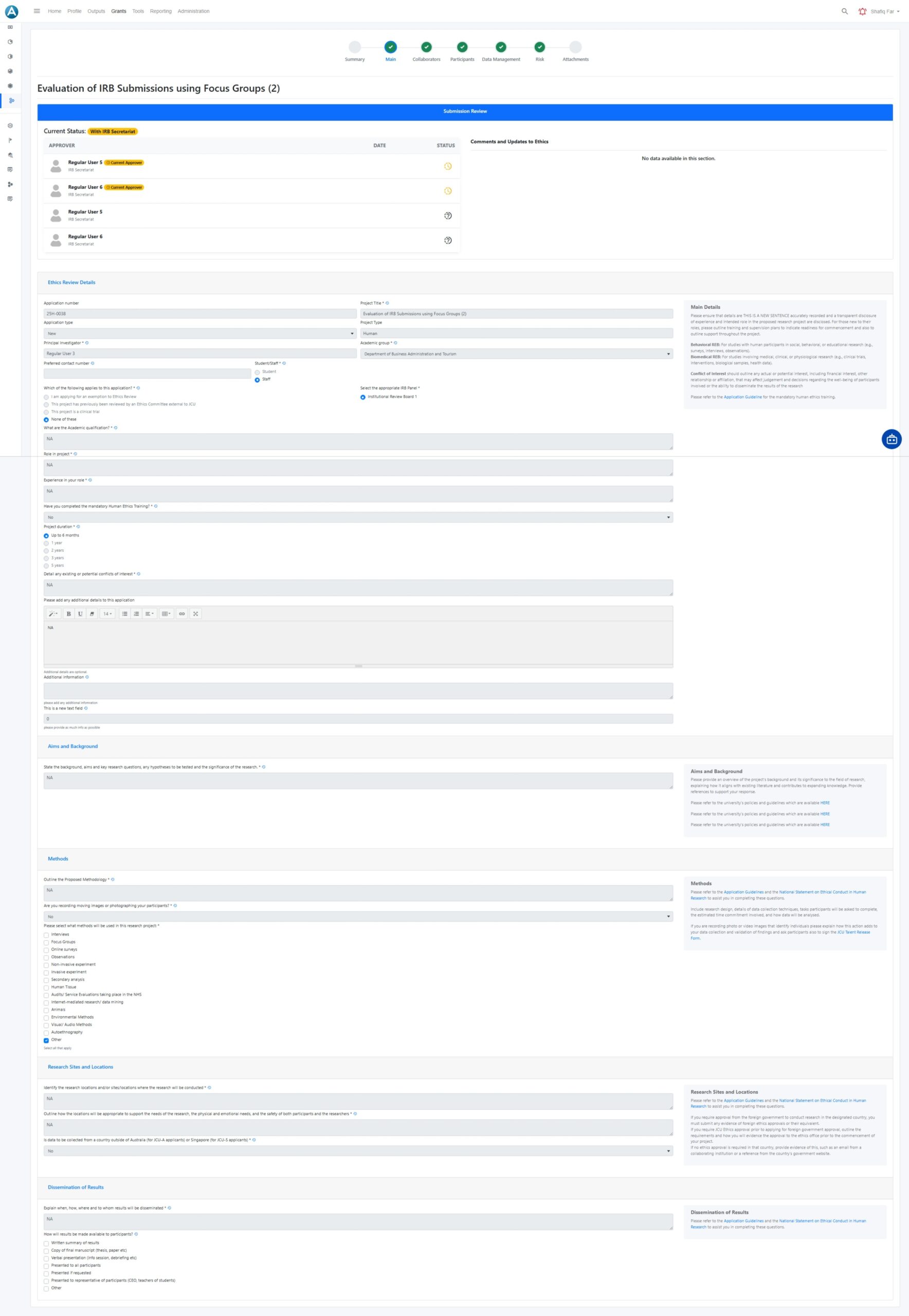
Ethics submission and review
Vidatum Ethics provides a structured environment for submitting, reviewing, and tracking ethics applications. Clear stages, role based access, and visible status updates help institutions maintain rigor while reducing delays and confusion.
- Guided ethics submissions with structured forms
- Clear status visibility across review stages
- Multi reviewer and committee based workflows
- Centralised communication and reviewer feedback
- Consistent handling of amendments and updates
Talk to the
Vidatum team
Discuss your institution’s research management needs and see how Vidatum supports confident, compliant processes across the full research lifecycle.

